999-97-3
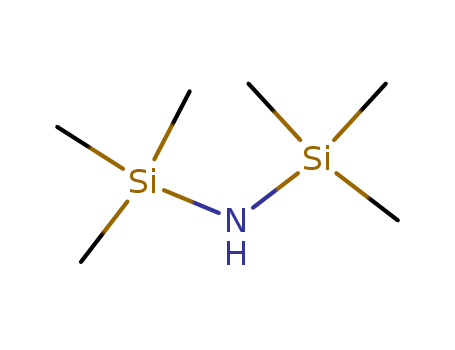
- Product Name:Hexamethyldisilazane(HMDZ)
- Molecular Formula:C6H19NSi2
- Purity:99%
- Molecular Weight:161.395
Product Details
Manufacturers supply cost-effective and customizable Hexamethyldisilazane(HMDZ) 999-97-3
- Molecular Formula:C6H19NSi2
- Molecular Weight:161.395
- Appearance/Colour:colourless liquid
- Vapor Pressure:20 hPa (20 °C)
- Melting Point:-78 °C
- Refractive Index:1.4069 - 1.4089
- Boiling Point:126 °C at 760 mmHg
- PKA:30(at 25℃)
- Flash Point:30 °C
- PSA:12.03000
- Density:0.776 g/cm3
- LogP:2.63670
Hexamethyldisilazane(Cas 999-97-3) Usage
|
Chemical Description |
Hexamethyldisilazane, trimethylchlorosilane, and pyridine are used to prepare the silylating reagent, which is used to treat the residue after the fractions are collected. |
|
Physical properties |
Colorless transparent and easy flowing liquid. Boiling point 125℃, relative density 0.76 (20/4℃). Soluble in organic solvents, it will be rapidly hydrolyzed in contact with air to form trimethylsilanol and hexamethyldisilyl ether. |
|
Definition |
ChEBI: Hexamethyldisilazane is an N-silyl compound obtained from ammonia by replacement of two of the hydrogens with trimethylsilyl groups. Hexamethyldisilazane is a derivatisation agent used in gas chromatography mass spectrometry applications. It has a role as a chromatographic reagent. It derives from a hydride of an ammonia. |
|
Reactions |
Hexamethyldisilazane(HMDS) is an extremely versatile and strong silylating agent. It is primarily used for the protection of sensitive functional groups during chemical synthesis.One of the advantages of Hexamethyldisilazane over chlorosilanes is that no base is needed as a hydrochloric acid acceptor. It produces ammonia which escapes easily from the reaction mixture during silylation. |
|
General Description |
A liquid. Boiling point of 260°F. Flash point 77°F. May be toxic by ingestion. Irritates skin and eyes. Vapors are heavier than air. May emit highly toxic nitrogen oxide fumes when heated to decomposition. Used to make other chemicals. |
|
Air & Water Reactions |
Highly flammable. Moisture sensitive. |
|
Reactivity Profile |
Hexamethyldisilazane reacts with many carbonyl containing organic compounds to generate gaseous ammonia. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. |
|
Health Hazard |
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
|
Flammability and Explosibility |
Flammable |
|
Purification Methods |
A possible impurity is Me3SiCl. Wash it well with pet ether and fractionate it through a vacuum jacketed column packed with Helipac using a reflux ratio of 10:1. [Langer et al. J Org Chem 23 50 1958, Beilstein 4 IV 4014.] |
InChI:InChI=1/C6H19NSi2/c1-8(2,3)7-9(4,5)6/h7H,1-6H3
999-97-3 Relevant articles
-
Wannagat,U. et al.
, p. 373 - 384 (1970)
-
From Ylides to Doubly Yldiide-Bridged Iron(II) High Spin Dimers via Self-Protolysis
Yogendra, Sivathmeehan,Weyhermüller, Thomas,Hahn, Anselm W.,Debeer, Serena
, p. 9358 - 9367 (2019)
A synthetic strategy for the preparation...
The Instability of Ni{N(SiMe3)2}2: A Fifty Year Old Transition Metal Silylamide Mystery
Faust, Michelle,Bryan, Aimee M.,Mansikkam?ki, Akseli,Vasko, Petra,Olmstead, Marilyn M.,Tuononen, Heikki M.,Grandjean, Fernande,Long, Gary J.,Power, Philip P.
, p. 12914 - 12917 (2015)
The characterization of the unstable NiI...
Ammonolysis of boron-substituted chlorosilylmethyl ortho-carborane derivatives
Izmailov,Qi, Shisheng,Markova,Vasnev
, p. 2338 - 2342 (2014)
We studied the ammonolysis of boron-subs...
Isolation and Reactivity Study of a Model 17-Electron Species in the Oxo Process
Takebayashi, Satoshi,Fayzullin, Robert R.
supporting information, p. 500 - 507 (2021/02/05)
[Co(L)(CO)3]2 (L = CO or PR3) catalyzed ...
Deoxygenation of primary amides to amines with pinacolborane catalyzed by Ca[N(SiMe3)2]2(THF)2
Gong, Mingliang,Guo, Chenjun,Jiang, Linhong,Luo, Yunjie,Yu, Chong
supporting information, p. 1201 - 1206 (2021/05/29)
Deoxygenative reduction of amides is a c...
Preparation method of silazane
-
Paragraph 0020-0021, (2020/10/14)
The invention provides a preparation met...
METHOD FOR PREPARING HYDROGEN BIS(FLUOROSULFONYL)IMIDE AND METHOD FOR PREPARING LITHIUM BIS(FLUOROSULFONYL)IMIDE
-
Paragraph 0051, (2020/06/07)
A method for preparing hydrogen bis(fluo...
999-97-3 Process route
-
-
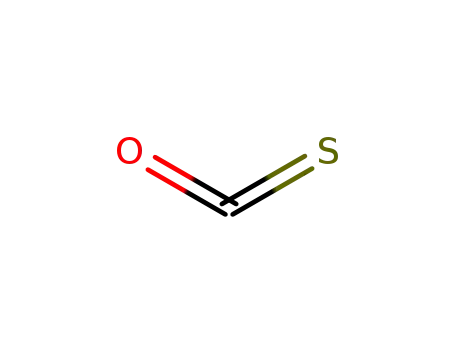
463-58-1
carbon oxide sulfide

-
-
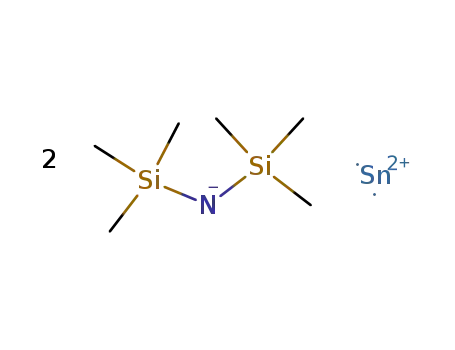
55147-78-9
bis(bis(trimethylsilyl)amido)tin(II)

-
-
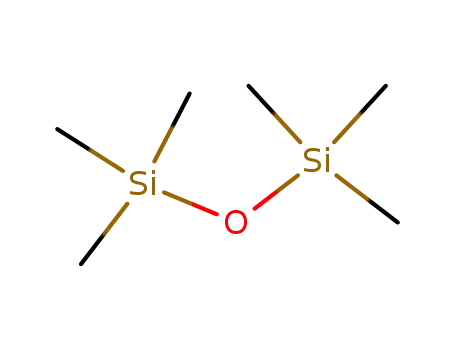
107-46-0
Hexamethyldisiloxane

-
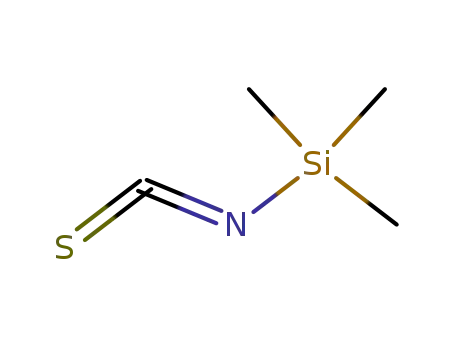
-
2290-65-5
trimethylsilyl isothiocyanate

-
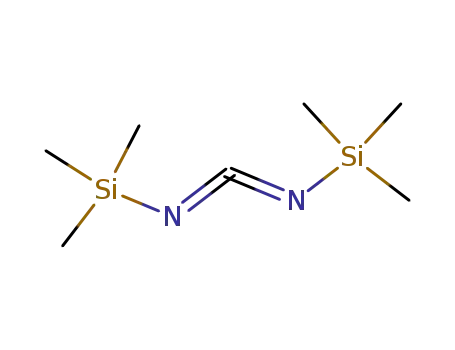
-
1000-70-0
bis(trimethylsilyl)carbodi-imide

-
-
Me3SiN(H)C(S)OSiMe3

-
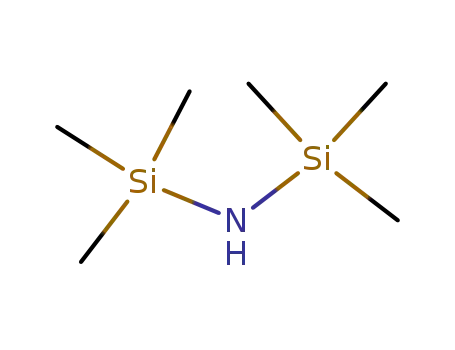
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane
| Conditions | Yield |
|---|---|
|
In
hexane;
at -100 - 25 ℃;
under 8000.32 Torr;
Inert atmosphere;
|
-

-
107-46-0
Hexamethyldisiloxane

-
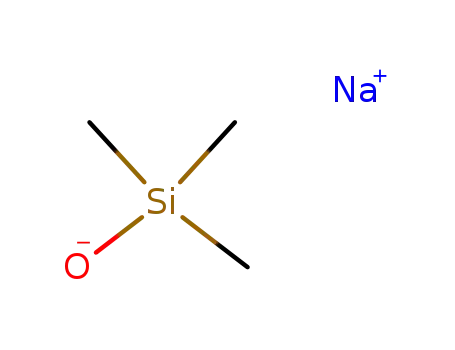
-
18027-10-6
sodium trimethylsilanolate

-

-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane
| Conditions | Yield |
|---|---|
|
With
Iron(III) nitrate nonahydrate; sodium amide;
In
tetrahydrofuran; 2-methyltetrahydrofuran;
at 60 - 66 ℃;
for 24h;
Concentration;
|
999-97-3 Upstream products
-
75-77-4
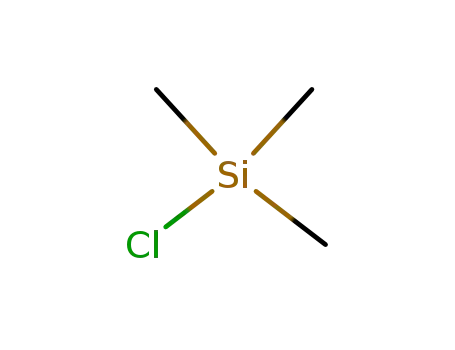
chloro-trimethyl-silane
-
18306-29-1
bis(trimethylsilyl)sulphate
-
109-99-9
tetrahydrofuran
-
67-56-1
methanol
999-97-3 Downstream products
-
7381-30-8
2,2,7,7-tetramethyl-3,6-dioxa-2,7-disilaoctane
-
10433-33-7
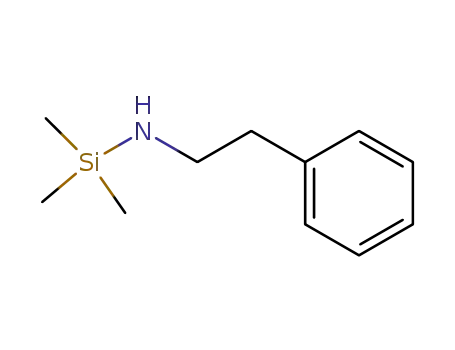
N-trimethylsilylphenethylamine
-
5577-66-2
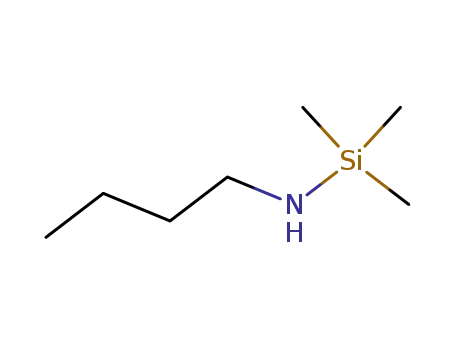
trimethyl(butyl-amino)silane
-
3768-55-6
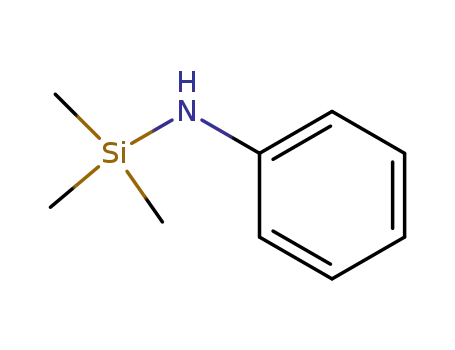
N-trimethylsilylaniline
Relevant Products
-
Diethylenetriaminopropylmethyldimethoxysilane
CAS:35141-30-1
-
1,1,3,3-Tetramethyldisilazane(TMDZ)
CAS:15933-59-2
-
Hexamethyldisilane(HMD)
CAS:1450-14-2