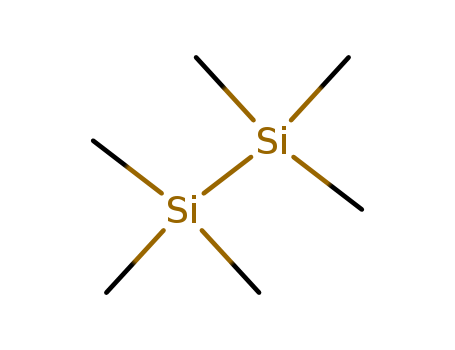
1450-14-2
- Product Name:Hexamethyldisilane(HMD)
- Molecular Formula:C6H18Si2
- Purity:99%
- Molecular Weight:146.38
Product Details
Good factory supply good Hexamethyldisilane(HMD) 1450-14-2
- Molecular Formula:C6H18Si2
- Molecular Weight:146.38
- Appearance/Colour:Colorless clear liquid
- Vapor Pressure:24.5mmHg at 25°C
- Melting Point:9-12 °C(lit.)
- Refractive Index:n20/D 1.422(lit.)
- Boiling Point:113.5 °C at 760 mmHg
- Flash Point:5.8 °C
- PSA:0.00000
- Density:0.735 g/cm3
- LogP:2.74120
Hexamethyldisilane(Cas 1450-14-2) Usage
|
Preparation |
Hexamethyldisilane is produced by the reaction of methyl Grignard reagent with methylchlorodisilanes. This reaction is normally conducted in the presence of an ether such as diethyl ether, ethyl propyl ether, THF and the like. |
|
Fire Hazard |
Hexamethyldisilazane (HMDZ) is a highly flammable liquid and vapour. HMDZ liquid is lighter than water and may float on water, spreading material during fire fighting. Leaking material or fire runoff to sewer may create fire or explosion hazard. HMDZ vapours are heavier than air and can accumulate to form explosive concentrations. The vapours may also spread a considerable distance to a source of ignition, and flash back towards the source. HMDZ reacts with water to form ammonia. HMDZ is a non-conductor and, therefore, can accumulate static electrical charges when processed, handled or dispensed. HMDZ's flash point is 52 to 53°F, closed cup, for "pure" material. |
|
Purification Methods |
The most likely impurity is trimethylchlorosilane (cf boiling point). Wash it with H2O, cold conc H2SO4, H2O again, then aqueous NaHCO3, dry over CaSO4 and fractionate at atmospheric pressure. [Brown & Fowles J Chem Soc 2811 1958.] A grossly impure sample (25% impurities) was purified by repeated spinning band distillation. This lowered the impurity level to 500ppm. The main impurity was identified as 1-hydroxypentamethyldisilane. [Beilstein 4 IV 4277.] |
|
Application |
Hexamethyldisilane is an important silane protective agent, which can synthesize sodium trimethylsilane, potassium trimethylsilane and lithium trimethylsilane.Preparation of iodotrimethylsilane.Used as trimethylsilane anion reagent.silylating or reducing reagent in combination with a Pd catalyst or a nucleophile.Replaces aromatic nitriles with TMS groups in presence of [RhCl(cod)]2.Precursor for CVD of silicon carbide.Brings about the homocoupling of arenesulfonyl chlorides in the presence of Pd2(dba)3.Used as a solvent for the direct borylation of fluoroaromatics.Reacts with alkynes to form siloles.Undergoes the silylation of acid chlorides to give acylsilanes. |
InChI:InChI=1/C6H18Si2/c1-7(2,3)8(4,5)6/h1-6H3
1450-14-2 Relevant articles
Disproportionation of Trimethylsilyl at 25 deg C. Mercury Photosensitization of Trimethylsilane
Tokach, S. K.,Koob, R. D.
, p. 376 - 377 (1980)
-
Catalytic Silylation of Dinitrogen with a Dicobalt Complex
Siedschlag, Randall B.,Bernales, Varinia,Vogiatzis, Konstantinos D.,Planas, Nora,Clouston, Laura J.,Bill, Eckhard,Gagliardi, Laura,Lu, Connie C.
, p. 4638 - 4641 (2015)
A dicobalt complex catalyzes N2 silylati...
Thermal SiSi/SiSi redistribution of hexaorganodisilane. A new thermally 'forbidden' molecular reaction
Sakurai, Hideki,Hosomi, Akira
, p. C15-C17 (1972)
-
-
Wilson,G.R.,Smith,A.G.
, p. 557 - 559 (1961)
-
Some Aspects of Chemistry of the N->Si Chelated Aryloxydihydrosilanes R(ArO)SiH2(R = Ph, ArO) and of the 2,2-Diaryloxytrisilane (Me3Si)2Si(OAr)2{ArO = 2,4,6-[(CH3)2NCH2]3C6H2O}
Ahdab, Aman Akkari-El,Rima, Ghassoub,Gornitzka, Heinz,Barrau, Jacques
, p. 94 - 105 (2002)
Reactions of the pentacoordinate aryldio...
L'electrosynthese, une voie simple d'acces aux di- et polysilanes
Biran, C.,Bordeau, M.,Pons, P.,Leger, M.-P.,Dunogues, J.
, p. C17 - C20 (1990)
Electrochemical reduction of chlorosilan...
Ultrasound in organometallic chemistry. The effects of temperature, metal purity and power source on the ultrasound-promoted reaction between trimethylchlorosilane and lithium
Lickiss, Paul D.,Lucas, Ronan
, p. 25 - 28 (1993)
The temperature, lithium purity, and the...
Intriguing tetrasodium dication cluster Na4/2+ stabilized between two silyl(fluorosilyl)phosphanide shells [8]
Driess,Pritzkow,Skipinski,Winkler
, p. 10774 - 10775 (1998)
-
Multiple-bond metathesis mediated by sterically pressured uranium complexes
Castro-Rodriguez, Ingrid,Nakai, Hidetaka,Meyer, Karsten
, p. 2389 - 2392 (2006)
(Figure Presented) Completing a circle o...
Molybdenum-catalyzed transformation of molecular dinitrogen into silylamine: Experimental and DFT study on the remarkable role of ferrocenyldiphosphine ligands
Tanaka, Hiromasa,Sasada, Akira,Kouno, Tomohisa,Yuki, Masahiro,Miyake, Yoshihiro,Nakanishi, Haruyuki,Nishibayashi, Yoshiaki,Yoshizawa, Kazunari
, p. 3498 - 3506 (2011)
A molybdenum-dinitrogen complex bearing ...
1: 2-Adducts from Silylenes and 1,3-butadiene
Bobbitt, Kevin L.,Gaspar, Peter P.
, p. 17 - 26 (1995)
Photochemical generation of dimethylsily...
-
Brockway,Davidson
, p. 3287,3289,3291 (1941)
-
Attenuation of Ni(0) Decomposition: Mechanistic Insights into AgF-Assisted Nickel-Mediated Silylation
Balakrishnan, Venkadesh,Chindan, Bincy,Murugesan, Vetrivelan,Rasappan, Ramesh
supporting information, p. 1438 - 1446 (2022/01/27)
In nickel-mediated Kumada cross-coupling...
Facile Synthesis of the Dicyanophosphide Anion via Electrochemical Activation of White Phosphorus: An Avenue to Organophosphorus Compounds
Liu, Liu Leo,Mei, Yanbo,Yan, Zeen
supporting information, p. 1517 - 1522 (2022/02/01)
Organophosphorus compounds (OPCs) have g...
Method for preparing hexamethyl disilane
-
Paragraph 0018-0037, (2019/07/04)
The invention discloses a method for pre...
Synthesis method of hexamethyl disilane
-
Paragraph 0013-0028, (2018/03/01)
The invention discloses a synthesis meth...
1450-14-2 Process route
-
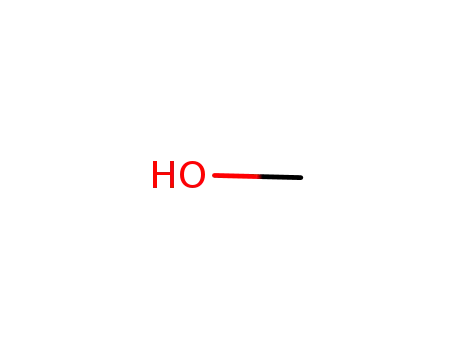
-
67-56-1
methanol

-
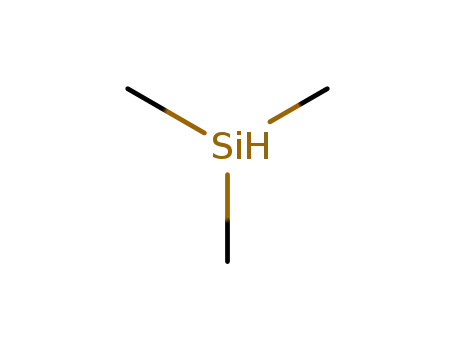
-
993-07-7
trimethylsilan

-
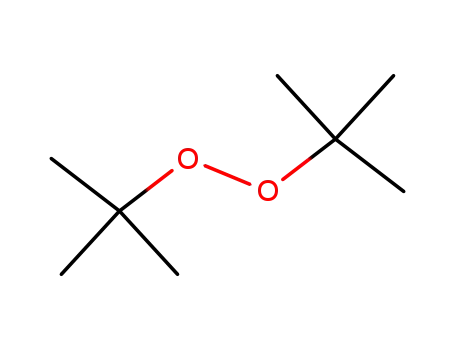
-
110-05-4
di-tert-butyl peroxide

-
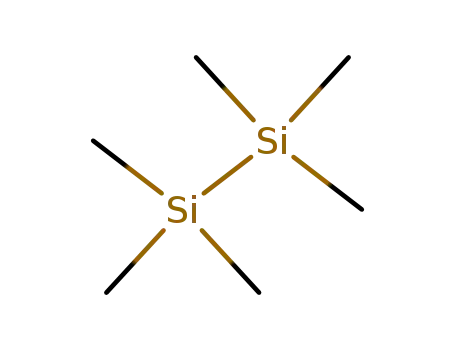
-
1450-14-2
1,1,1,2,2,2-hexamethyldisilane

-
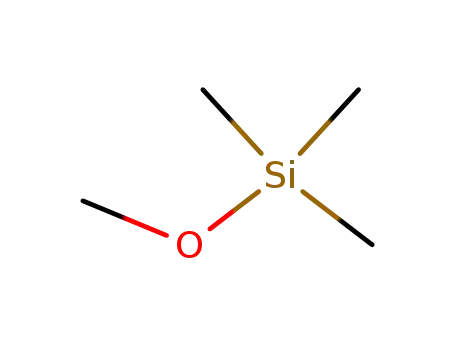
-
1825-61-2
Trimethylmethoxysilane
| Conditions | Yield |
|---|---|
|
Irradiation;
|
-
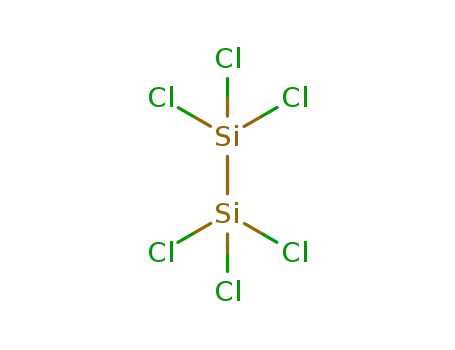
-
13465-77-5
hexachlorodisilane

-
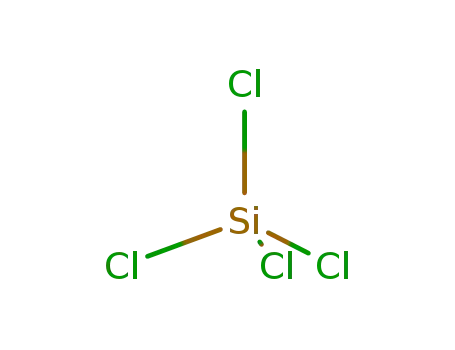
-
10026-04-7,53609-55-5
tetrachlorosilane

-
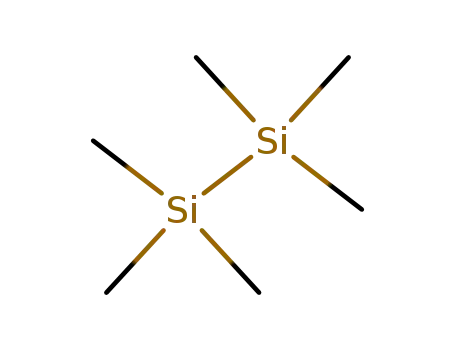
-
1450-14-2
1,1,1,2,2,2-hexamethyldisilane
| Conditions | Yield |
|---|---|
|
With
methylmagnesium bromide;
In
diethyl ether;
|
|
|
With
CH3MgBr;
In
diethyl ether;
|
1450-14-2 Upstream products
-
75-77-4
chloro-trimethyl-silane
-
3439-38-1

trimethylsilylethane
-
16029-98-4
trimethylsilyl iodide
-
768-32-1
trimethylphenylsilane
1450-14-2 Downstream products
-
2857-97-8
trimethylsilyl bromide
-
16029-98-4
trimethylsilyl iodide
-
1560-28-7
pentamethylchlorodisilane
-
4342-61-4
1,2-dichlorotetramethylsilane
Relevant Products
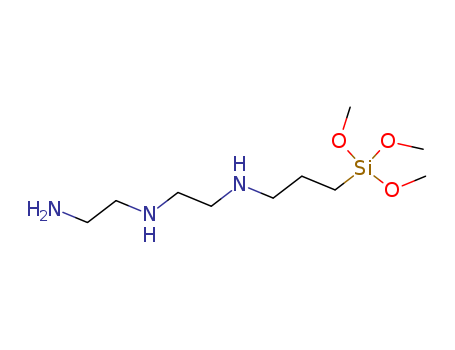
-
Diethylenetriaminopropylmethyldimethoxysilane
CAS:35141-30-1
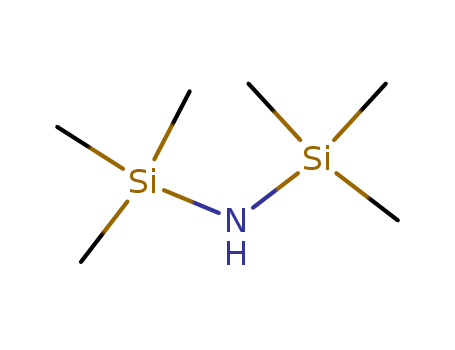
-
Hexamethyldisilazane(HMDZ)
CAS:999-97-3
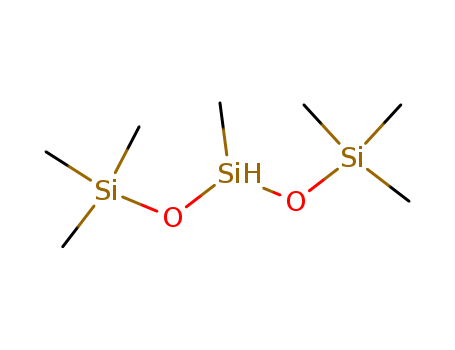
-
Heptamethyltrisiloxane(HPMTS)
CAS:1873-88-7