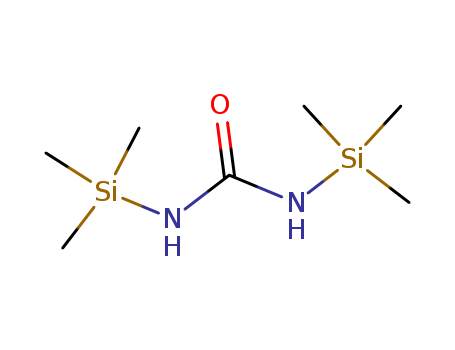
18297-63-7
- Product Name:Hexamethyldisilylurea(BSU)
- Molecular Formula:C7H20N2OSi2
- Purity:99%
- Molecular Weight:204.42
Product Details
Cost-effective and customizable Hexamethyldisilylurea(BSU) 18297-63-7 factory
- Molecular Formula:C7H20N2OSi2
- Molecular Weight:204.42
- Appearance/Colour:White solid with mild odor
- Vapor Pressure:0.177mmHg at 25°C
- Melting Point:219-221 °C(lit.)
- Refractive Index:1.442
- Boiling Point:222 °C
- PKA:16.51±0.46(Predicted)
- Flash Point:65 °C
- PSA:41.13000
- Density:0.887 g/cm3
- LogP:2.73720
1,3-Bis(trimethylsilyl)urea(Cas 18297-63-7) Usage
|
Flammability and Explosibility |
Notclassified |
|
General Description |
Silazane is any hydride of silicon and nitrogen having a straight or branched chain of silicon and nitrogen atoms joined by covalent bonds. By extension, the word is also used for any organic derivative of such compounds. They are analogous to siloxanes, with -NH- replacing -O-. Their individual name is dependent on the number of silicon atoms in the chemical structure. 1,3-Bis(trimethylsilyl)urea is an effective silylating agent. It is used in the pharmaceutical and in the chemical industry. |
InChI:InChI=1/C7H20N2OSi2/c1-11(2,3)9(7(8)10)12(4,5)6/h1-6H3,(H2,8,10)
18297-63-7 Relevant articles
Silica Metal Oxide Vesicles Catalyze Comprehensive Prebiotic Chemistry
Mattia Bizzarri, Bruno,Botta, Lorenzo,Pérez-Valverde, Maritza Iveth,Saladino, Raffaele,Di Mauro, Ernesto,García-Ruiz, Juan Manuel
, p. 8126 - 8132 (2018/05/29)
It has recently been demonstrated that m...
Synthesis and degradation of nucleic acid components by formamide and iron sulfur minerals
Saladino, Raffaele,Neri, Veronica,Crestini, Claudia,Costanzo, Giovanna,Graciotti, Michele,Di Mauro, Ernesto
experimental part, p. 15512 - 15518 (2009/03/12)
We describe the one-pot synthesis of a l...
Catalyst for use in production of epoxide, method for producing the catalyst, and method for producing epoxide
-
, (2008/06/13)
To provide an epoxide-production-use cat...
Carbacephalosporin compound, their preparation and use
-
, (2008/06/13)
Carbacephalosporin compounds of formula ...
18297-63-7 Process route
-
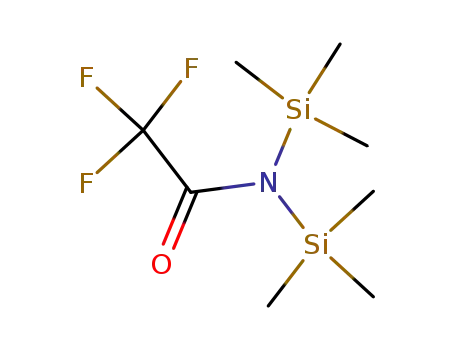
-
21149-38-2
2,2,2-trifluoro-N,N-bis(trimethylsilyl)-acetamide

-
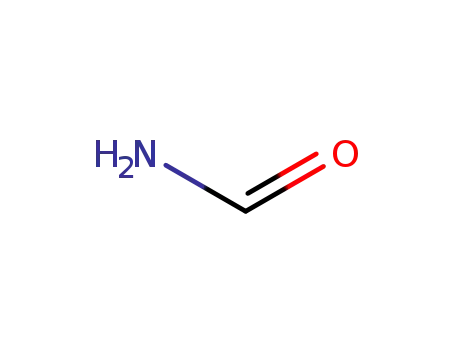
-
77287-34-4,77287-35-5,60100-09-6
formamide

-
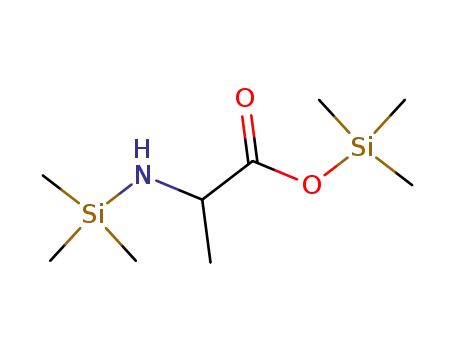
-
2899-44-7
N,O-bis(trimethylsilyl)-α-alanine

-
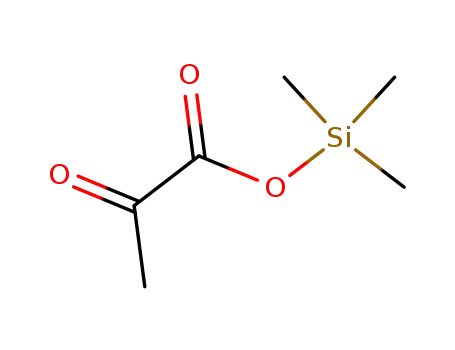
-
52060-85-2
2-methylglyoxylic acid trimethylsilyl ether

-
-
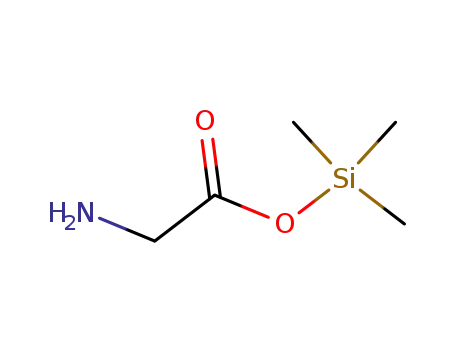
5269-37-4
glycine trimethylsilyl ester

-
-
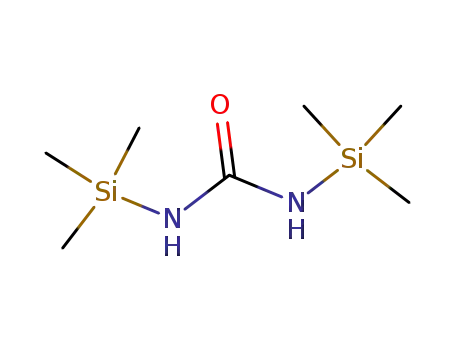
18297-63-7,127290-39-5
N,N'-bis(trimethylsilyl)urea

-
-
40309-57-7
butanedioic acid bis(trimethylsilyl) ester

-
![[(trimethylsilyl)oxy]butanedioic acid bis(trimethylsilyl) ester](/upload/2025/7/11841f20-3c34-48ff-86c8-0ef979b5ad96.png)
-
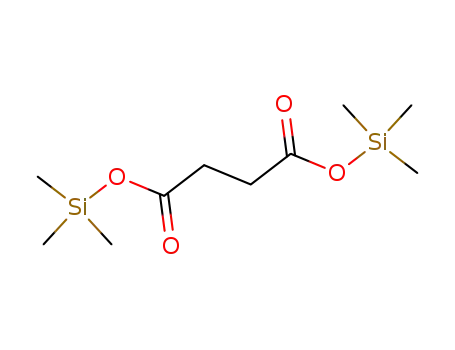
65143-63-7,107241-82-7,38166-11-9
[(trimethylsilyl)oxy]butanedioic acid bis(trimethylsilyl) ester

-
-
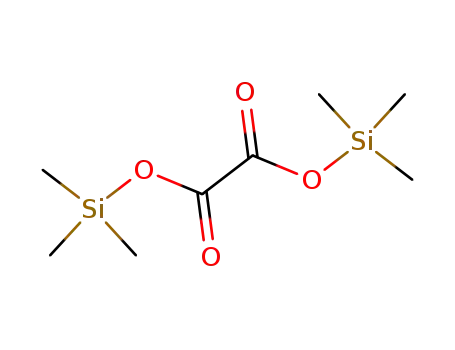
18294-04-7
ethanedioic acid bis(trimethylsilyl)ester

-
-
C11H20N4OSi2

-
-
C9H18N2O3Si2

-
-
C7H21N3Si2

-
-
C13H29N3OSi3

-
-
C8H21N5Si2

-
-
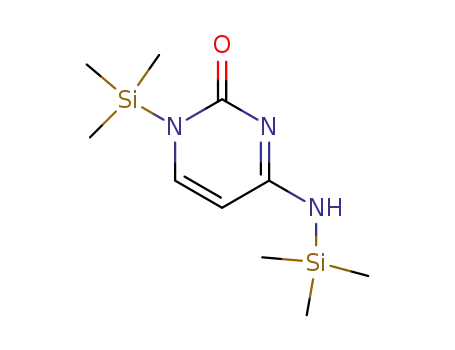
67014-25-9
bis(trimethylsilyl)cytosine

-
-
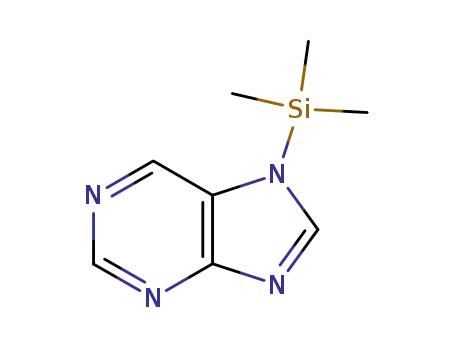
75773-52-3
7-Trimethylsilylpurin

-
-
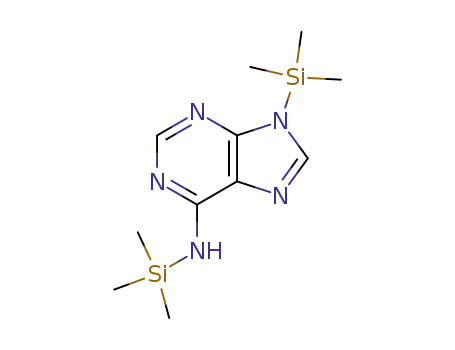
17995-04-9
N,N-bis(trimethysilyl)adenine

-
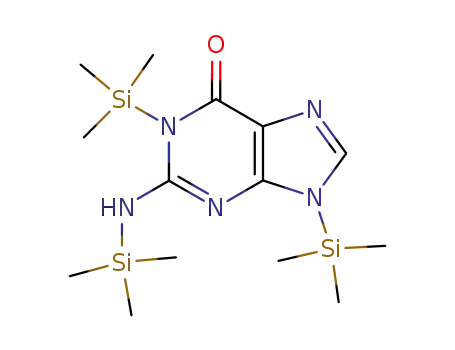
-
tris(trimethyl)guanine

-
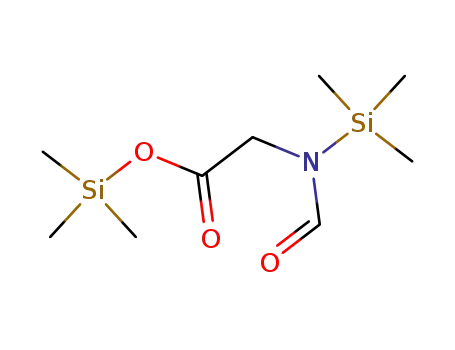
-
55517-31-2
N-Formyl-N,O-bis(trimethylsilyl)glycine

-
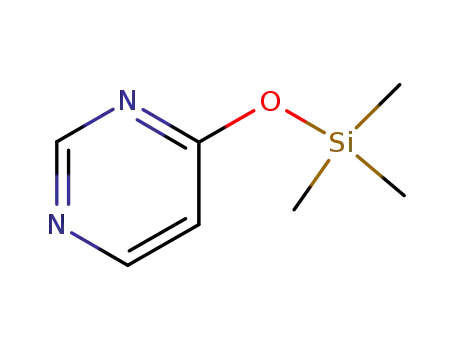
-
52523-25-8
4-(trimethylsiloxy)pyrimidine

-
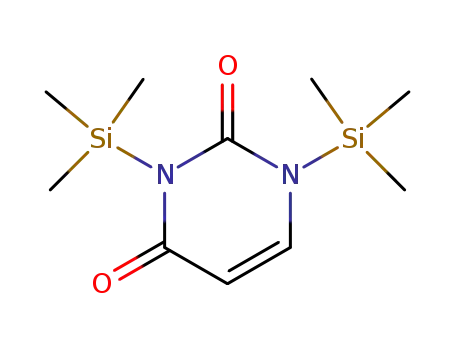
-
3442-82-8
1,3-bis(trimethylsilyl)uracil
| Conditions | Yield |
|---|---|
|
formamide;
With
water; magnesium sulfate;
at 80 ℃;
for 24h;
2,2,2-trifluoro-N,N-bis(trimethylsilyl)-acetamide;
With
pyridine;
at 60 ℃;
for 4h;
|
-

-
21149-38-2
2,2,2-trifluoro-N,N-bis(trimethylsilyl)-acetamide

-

-
77287-34-4,77287-35-5,60100-09-6
formamide

-

-
2899-44-7
N,O-bis(trimethylsilyl)-α-alanine

-

-
5269-37-4
glycine trimethylsilyl ester

-

-
18297-63-7,127290-39-5
N,N'-bis(trimethylsilyl)urea

-

-
40309-57-7
butanedioic acid bis(trimethylsilyl) ester

-
![propanoic acid,2-[(trimethylsilyl)oxy] trimethylsilyl ester](/upload/2025/7/4678ae3f-2475-4f95-aa00-8ec2c9a7011a.png)
-
17596-96-2
propanoic acid,2-[(trimethylsilyl)oxy] trimethylsilyl ester

-
![[(trimethylsilyl)oxy]butanedioic acid bis(trimethylsilyl) ester](/upload/2025/7/11841f20-3c34-48ff-86c8-0ef979b5ad96.png)
-
65143-63-7,107241-82-7,38166-11-9
[(trimethylsilyl)oxy]butanedioic acid bis(trimethylsilyl) ester

-

-
18294-04-7
ethanedioic acid bis(trimethylsilyl)ester

-
-
C11H20N4OSi2

-
-
C9H18N2O3Si2

-
-
C7H21N3Si2

-
-
C13H29N3OSi3

-
-
C10H22N4OSi2

-
-
C14H30N4O2Si3

-
-
C8H21N5Si2

-

-
67014-25-9
bis(trimethylsilyl)cytosine

-

-
75773-52-3
7-Trimethylsilylpurin

-

-
17995-04-9
N,N-bis(trimethysilyl)adenine

-

-
tris(trimethyl)guanine

-

-
55517-31-2
N-Formyl-N,O-bis(trimethylsilyl)glycine

-

-
52523-25-8
4-(trimethylsiloxy)pyrimidine

-

-
3442-82-8
1,3-bis(trimethylsilyl)uracil
| Conditions | Yield |
|---|---|
|
formamide;
With
ferric sulfate nonahydrate; water;
at 80 ℃;
for 24h;
2,2,2-trifluoro-N,N-bis(trimethylsilyl)-acetamide;
With
pyridine;
at 60 ℃;
for 4h;
|
18297-63-7 Upstream products
-
2083-91-2
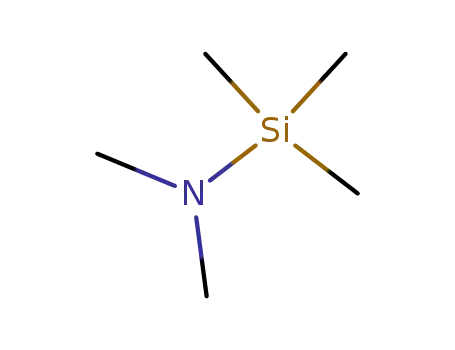
N,N-Dimethyltrimethylsilylamine
-
57-13-6
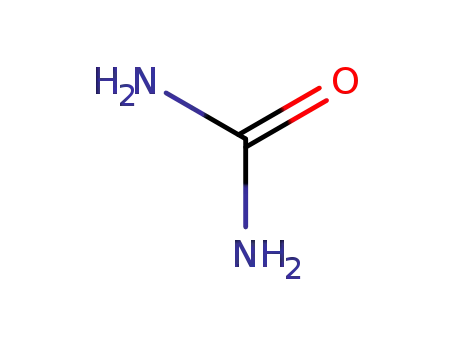
urea
-
999-97-3
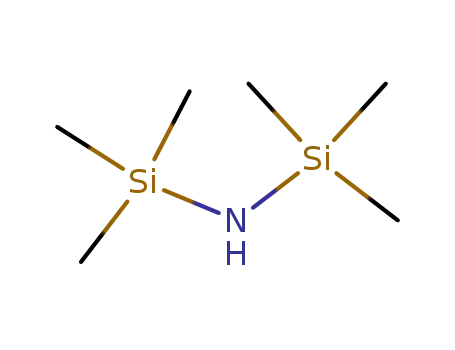
1,1,1,3,3,3-hexamethyl-disilazane
-
21149-38-2

2,2,2-trifluoro-N,N-bis(trimethylsilyl)-acetamide
18297-63-7 Downstream products
-
1000-70-0
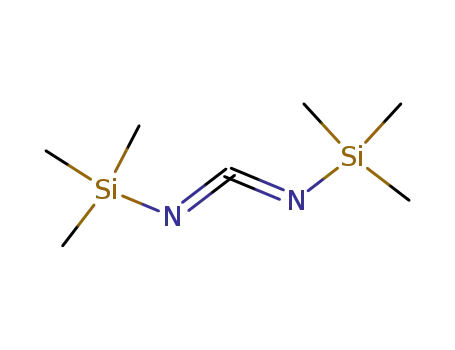
bis(trimethylsilyl)carbodi-imide
-
17902-32-8
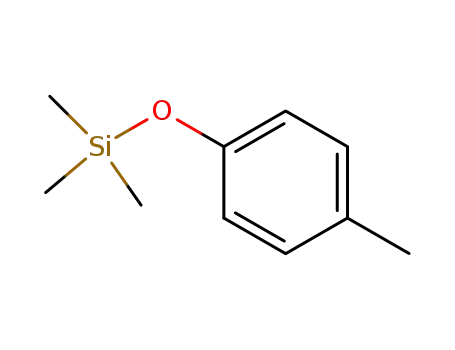
trimethyl(4-methylphenoxy)silane
-
13688-56-7
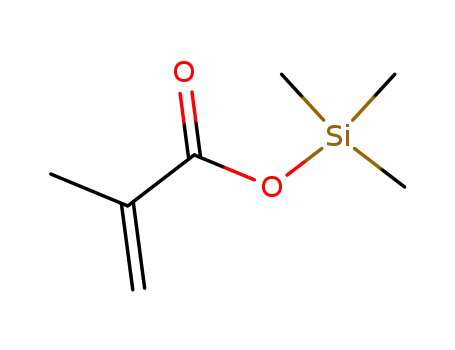
trimethylsilyl methacrylate
-
1856-05-9
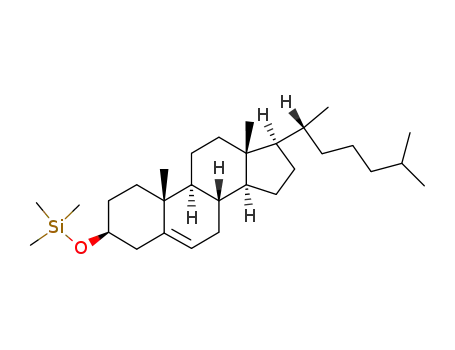
cholesterol trimethylsilyl ether
Relevant Products
-
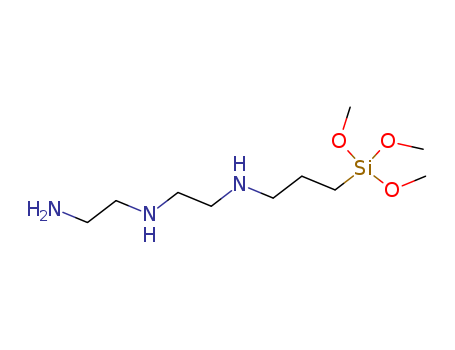
Diethylenetriaminopropylmethyldimethoxysilane
CAS:35141-30-1
-
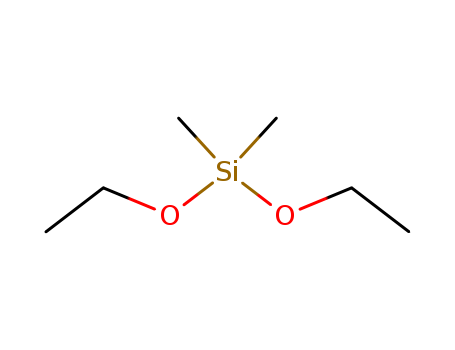
Dimethyldiethoxylsilane(DMDES)
CAS:78-62-6
-
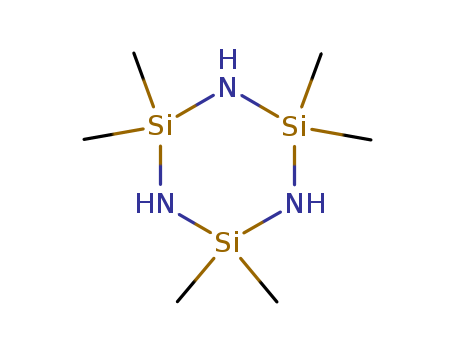
2,2,4,4,6,6-Hexamethylcyclotrisilazane(D3N)
CAS:1009-93-4