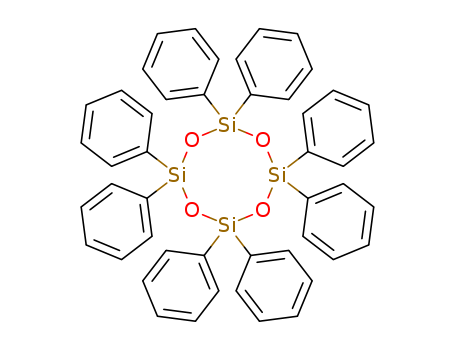
546-56-5
- Product Name:Octaphenylcyclotetrasiloxane(D4PH)
- Molecular Formula:C48H40O4Si4
- Purity:99%
- Molecular Weight:793.185
Product Details
Good factory exports good Octaphenylcyclotetrasiloxane(D4PH) 546-56-5
- Molecular Formula:C48H40O4Si4
- Molecular Weight:793.185
- Appearance/Colour:White solid
- Vapor Pressure:0Pa at 25℃
- Melting Point:196-198 °C(lit.)
- Refractive Index:1.66
- Boiling Point:334 °C
- Flash Point:200 °C
- PSA:36.92000
- Density:1.22 g/cm3
- LogP:5.09280
Octaphenylcyclotetrasiloxane(Cas 546-56-5) Usage
|
Preparation |
Octaphenylcyclotetrasiloxane is typically produced industrially by one of two processes: 1) the hydrolysis of diphenyldichlorosilane or 2) the hydrolysis of diphenyldialkoxysilanes.Process the production of octaphenylcyclotetrasiloxane |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Recrystallise it from AcOH, EtOAc, *C6H6 or *C6H6/EtOH. It forms two stable isomorphs and both forms, as well as the mixture, melt at 200-201o. There is a metastable form which melts at 187-189o. [Burkhard et al. J Am Chem Soc 67 2174 1945, Hyde et al. J Am Chem Soc 69 488 1947, Beilstein 16 IV 1530.] |
InChI:InChI=1/C48H40O4Si4/c1-9-25-41(26-10-1)53(42-27-11-2-12-28-42)49-54(43-29-13-3-14-30-43,44-31-15-4-16-32-44)51-56(47-37-21-7-22-38-47,48-39-23-8-24-40-48)52-55(50-53,45-33-17-5-18-34-45)46-35-19-6-20-36-46/h1-40H
546-56-5 Relevant articles
Divergent reactivity of divinylsilanes toward sulfonamides in different oxidative systems
Astakhova, Vera V.,Moskalik, Mikhail Yu.,Shainyan, Bagrat A.
, p. 40514 - 40528 (2020)
Oxidative sulfonamidation of divinylsila...
NHC-catalyzed dehydrogenative self-coupling of diphenylsilane: A facile synthesis of octaphenylcyclotetra(siloxane)
Albright, Abigail,Gawley, Robert E.
, p. 6130 - 6132 (2011)
A unique application of the CuIPr N-hete...
Reactivity of an Octanuclear Copper(I) Siloxide Compound - Isolation of a Copper(II) Oxo Compound with a Supertetrahedral Core
Schax, Fabian,Limberg, Christian
, p. 2060 - 2064 (2015)
The reactivity of the octanuclear copper...
A simple synthesis of octaphenylcyclotetra(siloxane)
Luo, Mei,Yan, Bing
, p. 5208 - 5209 (2009)
An essential industrial monomer octaphen...
Two cyclotetrasiloxanes at 143 K
Hensen, Karl,Gebhardt, Frank,Kettner, Markus,Pickel, Peter,Bolte, Michael
, p. 1867 - 1869 (1997)
The crystal structures of octaphenylcycl...
-
Burkhard,Decker,Harker
, p. 2174 ()
-
Synthesis and crystal structure of [K{O(Ph2SiO)2SiPh2OH}] 2·C6H6; the first structurally characterised example of a monometallated derivative of an α,ω-siloxane diol. Solution chemistry in relation to KOH-promoted ring-opening polymerisation of (Ph2SiO)3
Laermann, Barbara,Lazell, Michael,Motevalli, Majid,Sullivan, Alice C.
, p. 1263 - 1264 (1997)
The first structurally characterised exa...
Reactions of silicon hydrides catalyzed by rhodium(III) sulfoxide complexes
Eliseeva,Prudnikova,Panikorovskii,Skvortsov
, p. 1884 - 1886 (2017)
Dehydrocondensation reactions of silicon...
Cobalt-Catalyzed Selective Synthesis of Disiloxanes and Hydrodisiloxanes
Pattanaik, Sandip,Gunanathan, Chidambaram
, p. 5552 - 5561 (2019/06/05)
Selective syntheses of symmetrical silox...
Renewable Isohexide-Based, Hydrolytically Degradable Poly(silyl ether)s with High Thermal Stability
Vijjamarri, Srikanth,Hull, Marianne,Kolodka, Edward,Du, Guodong
, p. 2881 - 2888 (2018/09/18)
Several degradable poly(silyl ether)s (P...
Controlled synthesis of cyclosiloxanes by NHC-catalyzed hydrolytic oxidation of dihydrosilanes
Qing, Guoping,Cui, Chunming
, p. 8746 - 8750 (2017/07/22)
Hydrolytic oxidation of various hydrosil...
546-56-5 Process route
-

- 775-12-2
diphenylsilane

-
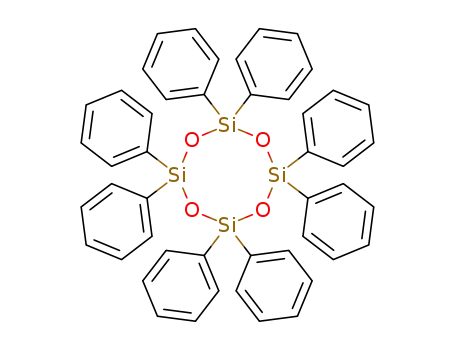
- 546-56-5
octaphenylcyclotetrasiloxane
| Conditions | Yield |
|---|---|
|
With oxygen; sodium t-butanolate; In tetrahydrofuran; at 20 ℃; for 1h;
|
100% |
|
With C15H27Br2CoN3; potassium tert-butylate; water; In 1,4-dioxane; at 60 ℃; for 2h; Reagent/catalyst;
|
96% |
|
With water; 2,3-Dihydro-1,3-diisopropyl-4,5-dimethylimidazol-2-ylidene; In acetonitrile; at 20 ℃; for 2.5h; Time; Solvent; Concentration; Schlenk technique; Glovebox;
|
92% |
|
With [Rh(dimethylsulfoxide)3Cl3]; at 40 - 50 ℃;
|
-
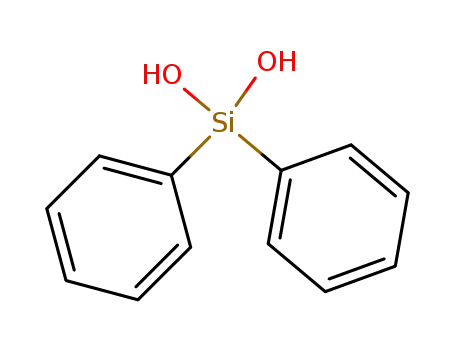
- 947-42-2
diphenylsilanediol

-

- 546-56-5
octaphenylcyclotetrasiloxane
| Conditions | Yield |
|---|---|
|
With triethylamine; In toluene;
|
92.1% |
|
at 140 - 180 ℃;
|
|
|
With acetyl chloride;
|
|
|
With piperidine;
|
|
|
With sodium hydroxide; ethanol;
|
|
|
With sodium hydroxide; In ethanol; for 0.5h;
|
88 % Chromat. |
|
Multi-step reaction with 2 steps
1: hydrochloric acid
2: acetyl chloride
With hydrogenchloride; acetyl chloride;
|
|
|
Multi-step reaction with 2 steps
1: not given
2: CH3COCl / further solvent(s)
With CH3COCl; In not given; further solvent(s);
|
|
|
Multi-step reaction with 2 steps
1: not given
2: neat (no solvent)
In not given; neat (no solvent);
|
|
|
Multi-step reaction with 2 steps
2: CH3COCl / further solvent(s)
With CH3COCl; In further solvent(s);
|
|
|
Multi-step reaction with 2 steps
2: neat (no solvent)
In neat (no solvent);
|
|
|
With H2O; NH3; In ethanol; addn. of a small amt. of aq. NH3 to (C6H5)2Si(OH)2 in hot ethanol;; ppt. on cooling;;
|
|
|
With NaOH; In ethanol; addn. of a small amt. of NaOH-soln. to (C6H5)2Si(OH)2 in hot ethanol;; ppt. on cooling;;
|
|
|
In not given; (C6H5)2Si(OH)2 in alkaline soln.;;
|
546-56-5 Upstream products
-
110-89-4
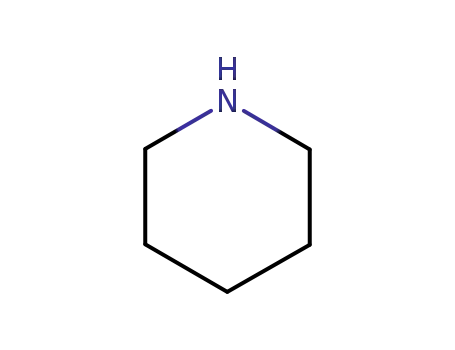
piperidine
-
60-29-7
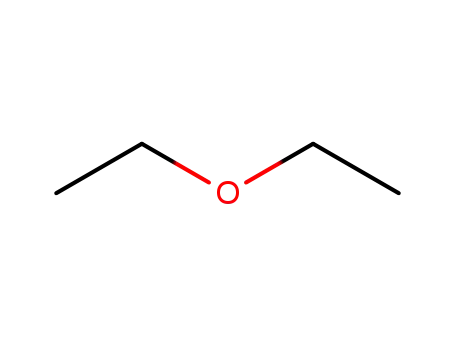
diethyl ether
-
1104-93-4
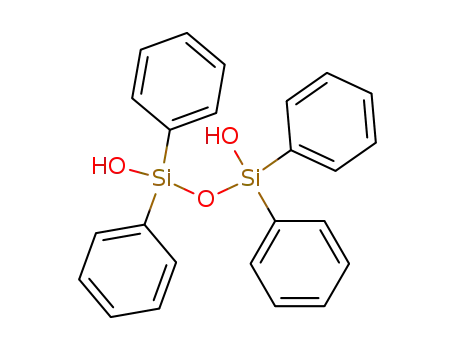
1,1,3,3-tetraphenyldisiloxane-1,3-diol
-
1110-85-6
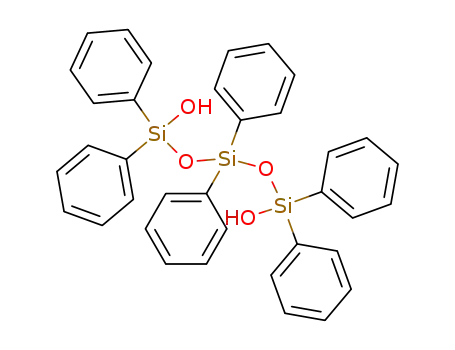
Hexaphenyltrisiloxan-1,5-diol
546-56-5 Downstream products
-
18840-65-8
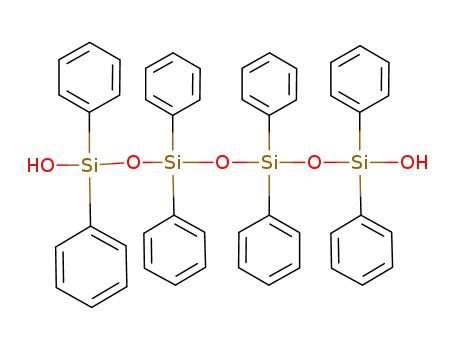
1,1,3,3,5,5,7,7-octaphenyl-1,3,5,7-tetrasiloxane-1,7-diol
-
18755-09-4
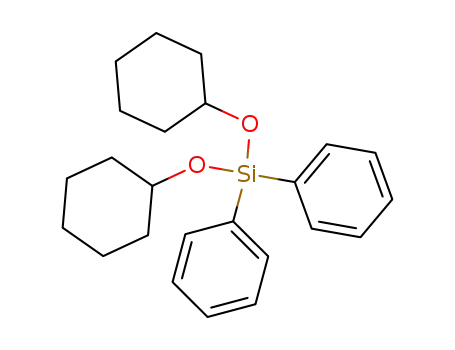
bis-cyclohexyloxy-diphenyl-silane
-
312-40-3
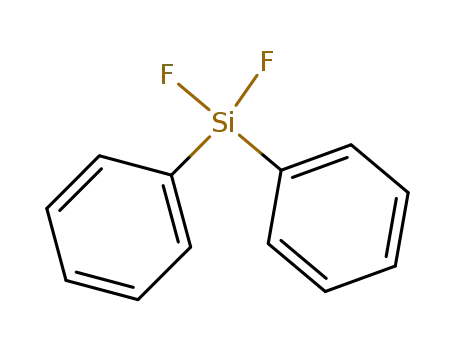
difluorodiphenylsilane
-
26599-04-2
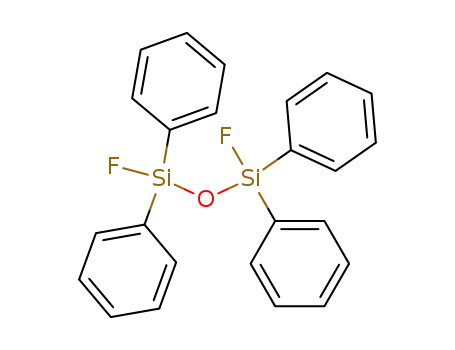
1,3-difluoro-1,1,3,3,-tetraphenyldisiloxane
Relevant Products
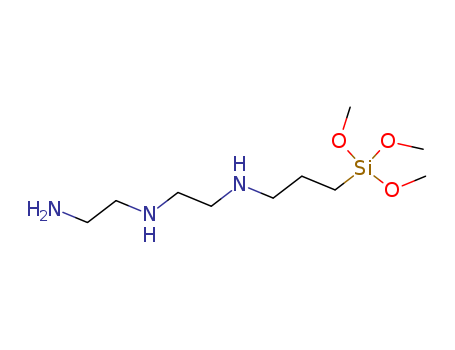
-
Diethylenetriaminopropylmethyldimethoxysilane
CAS:35141-30-1
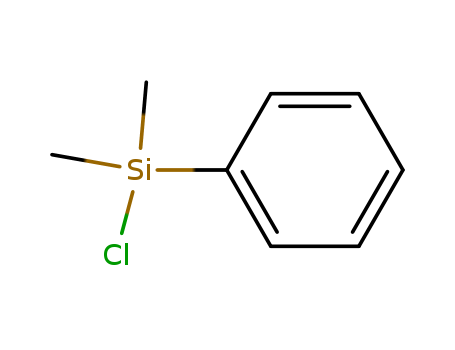
-
Chlorodimethylphenylsilane(DMPCS)
CAS:768-33-2
-
PHENYLMETHYLCYCLOSILOXANE(MPCS)
CAS:68037-54-7