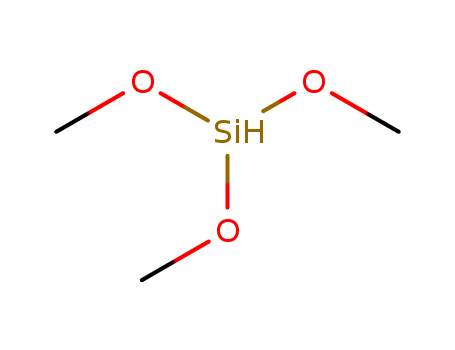
2487-90-3
- Product Name:Trimethoxysilane
- Molecular Formula:C3H10O3Si
- Purity:99%
- Molecular Weight:122.196
Product Details
China cas 2487-90-3 manufacturer wholesale Trimethoxysilane at affordable price
- Molecular Formula:C3H10O3Si
- Molecular Weight:122.196
- Appearance/Colour:colorless transparent liquid
- Vapor Pressure:<7.2 mm Hg ( 20 °C)
- Melting Point:-115 °C(lit.)
- Refractive Index:n20/D 1.358(lit.)
- Boiling Point:81 °C at 760 mmHg
- Flash Point:24 °F
- PSA:27.69000
- Density:0.96 g/cm3
- LogP:-0.35720
Trimethoxysilane(Cas 2487-90-3) Usage
|
Air & Water Reactions |
Highly flammable. Slightly soluble in water. |
|
Reactivity Profile |
Trimethoxysilane may accumulate static electricity; hazardous polymerization may occur. |
|
Health Hazard |
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution. |
|
Fire Hazard |
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
|
Flammability and Explosibility |
Flammable |
|
Safety Profile |
Moderately toxic by inhalation. Mildly toxic by ingestion and skin contact. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Purification Methods |
Likely impurities are Si(OMe)4 and H2Si(OMe)2. Efficient fractionation is essential for removing these impurities [IR: Sternbach & MacDiarmid J Am Chem Soc 81 5109 1959, Heilfrich & Hausen Chem Ber 57 795 1924, Beilstein 1 IV 1266.] |
|
General Description |
A colorless liquid. Slightly soluble in water and denser than water. Hence floats on water. Very toxic by ingestion, inhalation or skin absorption. May also be corrosive to skin and eyes. |
InChI:InChI=1/C3H9O3Si/c1-4-7(5-2)6-3/h1-3H3
2487-90-3 Relevant articles
Direct Synthesis of Trimethoxysilane from Methanol and Hydrogen-Treated Silicon Using Copper(II) Chloride as a Catalyst
Suzuki, Eiichi,Kamata, Takatsugu,Ono, Yoshio
, p. 3445 - 3447 (1991)
When silicon, on which copper(II) chlori...
The direct synthesis of organic derivatives of silicon using nonhalogenated organic compounds
Newton,Rochow
, p. 1071 - 1075 (1970)
Some trialkoxysilanes, (RO)3SiH (R = CH3...
A Kinetic Study on the Copper-catalyzed Reaction of Silicon with Methanol into Trimethoxysilane
Suzuki, Eiichi,Okamoto, Masaki,Ono, Yoshio
, p. 199 - 202 (1991)
The reaction of methanol with silicon wa...
Hydrosilylation of ethylene
Chernyshev,Belyakova,Knyazev,Turkel'taub,Parshina,Serova,Storozhenko
, p. 225 - 228 (2006)
Hydrosilylation of ethylene with trialko...
Direct reaction between silicon and methanol over Cu-based catalysts: Investigation of active species and regeneration of CuCl catalyst
Wang, Aili,Zhang, Mingming,Yin, Hengbo,Liu, Shuxin,Liu, Mengke,Hu, Tongjie
, p. 19317 - 19325 (2018/05/31)
When a CuCl/Si mixture was pretreated at...
A three-methoxy silane synthesis method (by machine translation)
-
Paragraph 0026-0029; 0046; 0047, (2017/08/27)
The invention provides a three-methoxy s...
New vinyl alkoxy silane preparation process
-
Paragraph 0037; 0038, (2016/10/08)
The present invention discloses a new vi...
Method for synthesizing trimethoxy silane through fixed bed reaction
-
Paragraph 0018-0020; 0023-0024; 0027-0028; 0031-0032, (2017/08/25)
The invention relates to the technical f...
2487-90-3 Process route
-
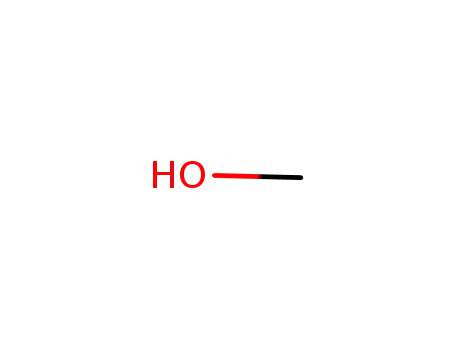
- 67-56-1
methanol

-
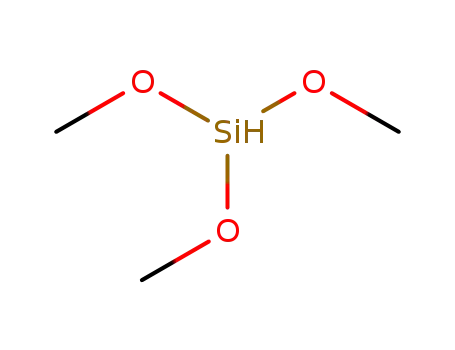
- 2487-90-3
trimethoxysilane
| Conditions | Yield |
|---|---|
|
With copper(II) nitrate trihydrate; cetyltrimethylammonim bromide; hydrazine hydrate; silicon; at 220 - 260 ℃; for 1h; under 760.051 Torr; Microwave irradiation;
|
96.9% |
|
With trichlorosilane; at 100 ℃; for 6h; Temperature;
|
82.6% |
|
With amorphous silicon; In paraffin oil; at 230 ℃; for 5h; Inert atmosphere; Schlenk technique;
|
33% |
|
With trichlorosilane;
|
|
|
With silicon;
|
|
|
With silica powder-catalyst; at 220 ℃; for 1h; under 760.051 Torr; Temperature; Inert atmosphere;
|
-

- 67-56-1
methanol

-

- 7440-21-3
silicon

-

- 2487-90-3
trimethoxysilane

-
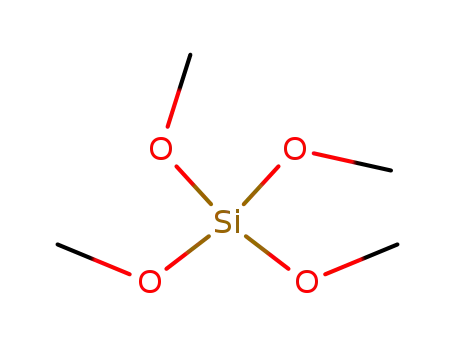
- 681-84-5
tetramethylorthosilicate
| Conditions | Yield |
|---|---|
|
copper(II) hydroxide; In THERMINOL 59; at 250 ℃; Product distribution / selectivity;
|
|
|
basic copper phosphate; In THERMINOL 59; at 250 ℃; Product distribution / selectivity;
|
|
|
copper(II) dimetaphosphate; In THERMINOL 59; at 250 ℃; Product distribution / selectivity;
|
|
|
In THERMINOL 59; at 250 ℃; Product distribution / selectivity;
|
|
|
With FS1265; copper(II) hydroxide; In THERMINOL 59; at 250 ℃; for 14.78h; Product distribution / selectivity;
|
2487-90-3 Upstream products
-
67-56-1

methanol
-
1471-03-0
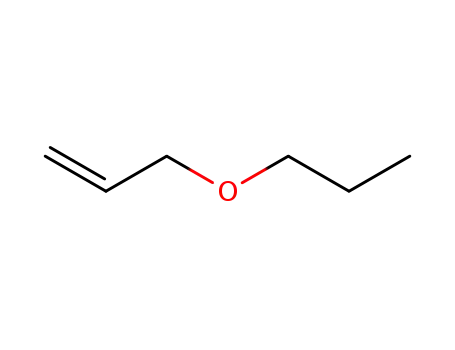
allyl propyl ether
-
74-85-1
ethene
-
124-41-4
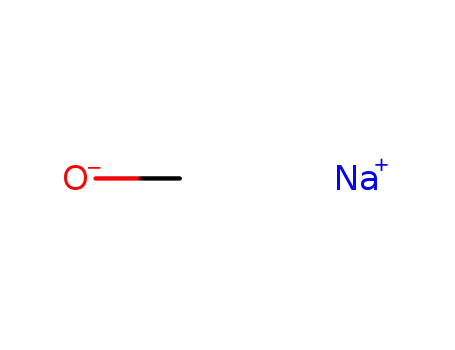
sodium methylate
2487-90-3 Downstream products
-
17805-80-0
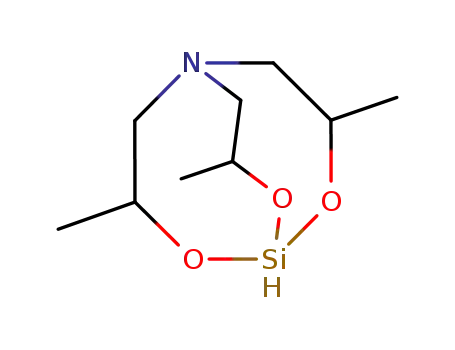
3,7,10-Trimethylsilatran
-
3069-19-0
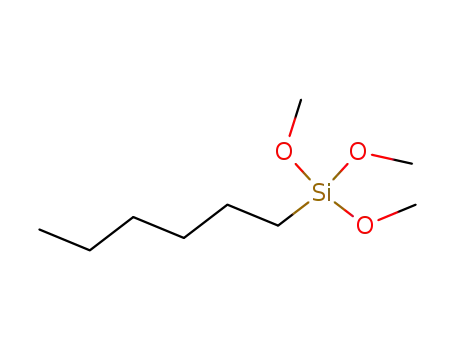
n-hexyltrimethoxysilane
-
283-60-3
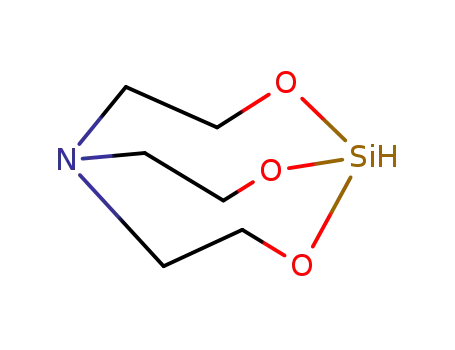
silatrane
-
25760-57-0
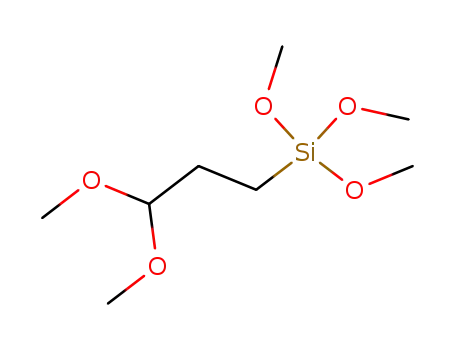
(3,3-dimethoxypropyl)trimethoxysilane
Relevant Products
-
Diethylenetriaminopropylmethyldimethoxysilane
CAS:35141-30-1
-
Tetraethoxysilane-28
CAS:78-10-4
-
Vinyltriethoxysilane
CAS:78-08-0