2083-91-2
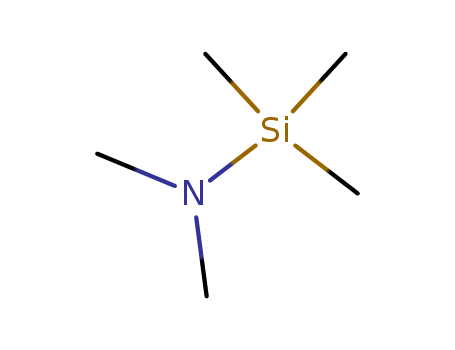
- Product Name:N,N-Dimethyltrimethylsilylamine(DMTMSA)
- Molecular Formula:C5H15NSi
- Purity:99%
- Molecular Weight:117.266
Product Details
Manufacturers supply cost-effective and customizable N,N-Dimethyltrimethylsilylamine(DMTMSA) 2083-91-2
- Molecular Formula:C5H15NSi
- Molecular Weight:117.266
- Appearance/Colour:clear colorless to pale yellow liquid
- Vapor Pressure:4.11mmHg at 25°C
- Melting Point:<0 °C
- Refractive Index:n20/D 1.397(lit.)
- Boiling Point:89.3 °C at 760 mmHg
- PKA:10.77±0.70(Predicted)
- Flash Point:-19 °C
- PSA:3.24000
- Density:0.761 g/cm3
- LogP:1.38290
N,N-Dimethyltrimethylsilylamine(Cas 2083-91-2) Usage
|
General Description |
N,N-Dimethyltrimethylsilylamine (Me3SiNMe2) is a silylamine compound formed as the primary product in the reaction of iodo(trimethyl)silane (Me3SiI) with N,N-dimethylformamide (DMF) or N,N-dimethylacetamide (DMA), resulting from the cleavage of the N–C(=O) bond. It is accompanied by the generation of acyl iodides (RCOI) and minor by-products such as N-methyl-N-trimethylsilyl derivatives and N-methyl imides. The reaction proceeds via an intermediate quaternary ammonium salt, which decomposes to yield Me3SiNMe2 with high efficiency (83% for DMF and 77% for DMA). |
InChI:InChI=1/C7H21NSi2/c1-8(9(2,3)4)10(5,6)7/h1-7H3
2083-91-2 Relevant articles
Latent Nucleophilic Carbenes
Hurieva, Anastasiya,Koidan, Georgyi,Kostyuk, Aleksandr,Kyrylchuk, Andrii A.,Marchenko, Anatoliy,Rozhenko, Alexander B.,Rusanov, Eduard B.,Shvydenko, Kostiantyn
, (2021/12/27)
Using DFT and ab initio calculations, we...
IMINE-TYPE QUATERNARY AMMONIUM SALT CATALYST, PREPARATION METHOD THEREOF AND USE THEREOF FOR PREPARATION OF POLYISOCYANATE COMPOSITION
-
Paragraph 0021; 0057-0058; 0077-0078, (2020/12/13)
Disclosed is an imine-type quaternary am...
METHOD FOR PRODUCING DIALKYLAMINOSILANE
-
Paragraph 0044-0048, (2018/03/09)
In a method for synthesizing dialkylamin...
An Alumino-Mannich Reaction of Organoaluminum Reagents, Silylated Amines, and Aldehydes
Tarasewicz, Anika,Ensan, Deeba,Batey, Robert A.
supporting information, p. 6071 - 6074 (2018/04/27)
A multi-component coupling using organoa...
2083-91-2 Process route
-
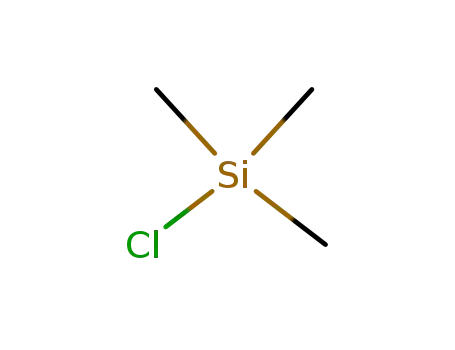
-
75-77-4
chloro-trimethyl-silane

-
-
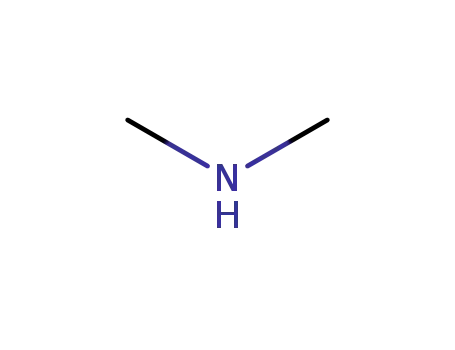
124-40-3
dimethyl amine

-
-
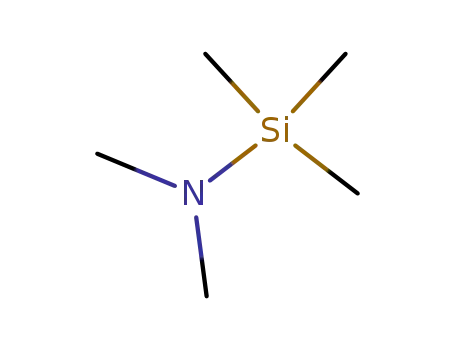
2083-91-2
N,N-Dimethyltrimethylsilylamine
| Conditions | Yield |
|---|---|
|
In
methylbutane;
Ambient temperature;
|
75% |
|
In
octane; acetonitrile;
at 20 ℃;
for 5h;
Solvent;
Reflux;
|
70% |
|
|
|
|
In
xylene;
|
|
|
In
xylene;
|
|
|
With
triethylamine;
In
dichloromethane;
at 20 ℃;
for 18h;
Inert atmosphere;
|
|
|
at 20 ℃;
for 0.5h;
Cooling with ice;
|
-

-
124-40-3
dimethyl amine

-
-
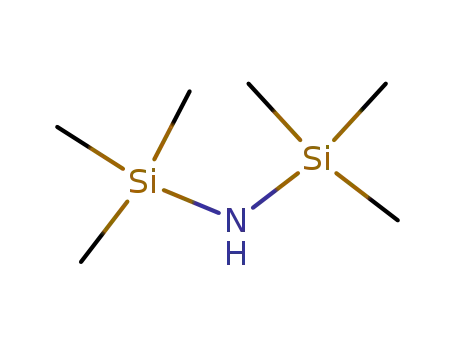
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-

-
2083-91-2
N,N-Dimethyltrimethylsilylamine
| Conditions | Yield |
|---|---|
|
With
1,3-dimethyl-2-imidazolidinone;
at 40 ℃;
Reagent/catalyst;
|
94% |
|
With
ammonium sulfate;
at 80 ℃;
for 8h;
Reagent/catalyst;
Time;
Autoclave;
|
84% |
2083-91-2 Upstream products
-
75-77-4
chloro-trimethyl-silane
-
124-40-3

dimethyl amine
-
18135-05-2
N-(trimethylsilylmethyl)methylamine
-
14937-39-4
methyl-bis(dimethylamino)phosphine
2083-91-2 Downstream products
-
420-56-4
trimethylsilyl fluoride
-
23184-28-3
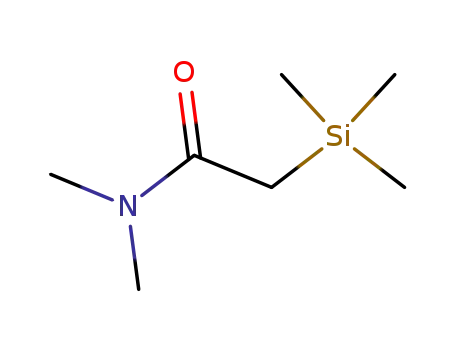
N,N-dimethyl(trimethylsilyl)acetamide
-
39055-14-6
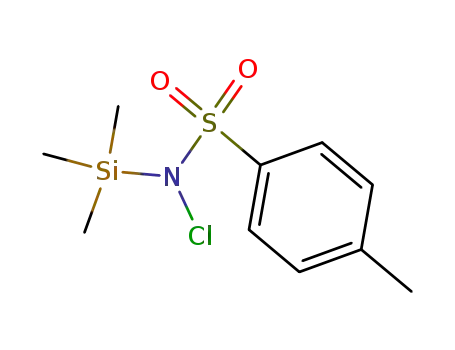
N-chloro-N-trimethylsilyl-4-toluenesulfonamide
-
1857-20-1
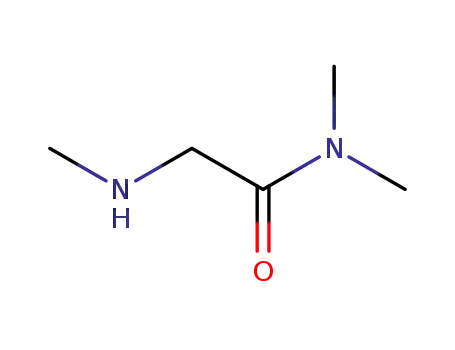
sarcosine dimethylamide
Relevant Products
-
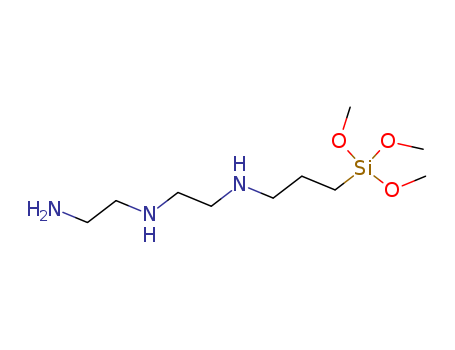
Diethylenetriaminopropylmethyldimethoxysilane
CAS:35141-30-1
-
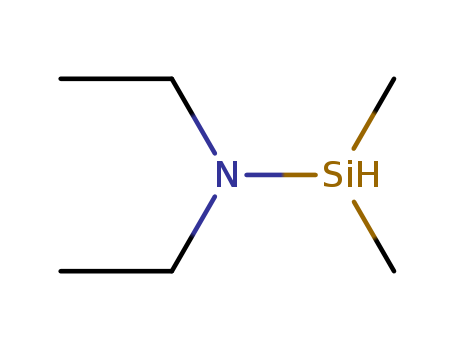
Dimethylsilyldiethylamine(DMSDEA)
CAS:13686-66-3
-
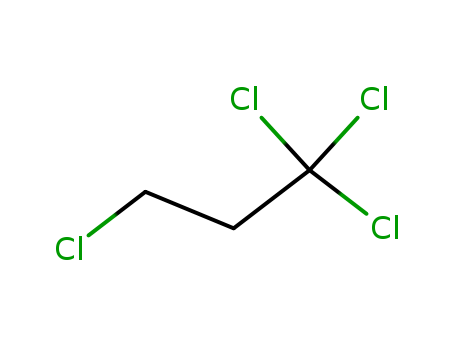
1,1,1,3-Tetrachloropropane
CAS:1070-78-6