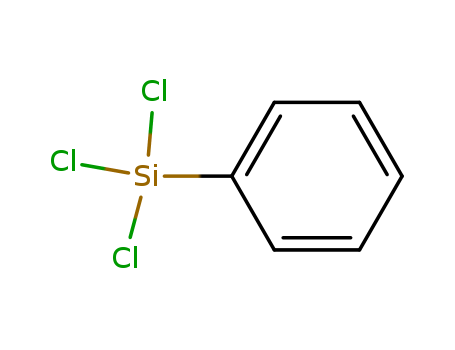
98-13-5
- Product Name:Phenyltrichlorosilane(PTCS)
- Molecular Formula:C6H5Cl3Si
- Purity:98%
- Molecular Weight:211.55
Product Details
Phenyltrichlorosilane (PTCS/P1)
| Molecular Formula | C6H5C13Si | Molecular Weight | 211.55 |
| Cas no. | 98-13-5 | UN no. | 1804 |
| Production criterion code | GB/T 30302-2013 | Appearance | Tansparent Liquid |
Properties Index:
| Assay: | ≥98.0% | Spedific gravite (25 ℃) | 1.321g/cm3 |
| Bolling Point: | 201℃ | Flash Point: | 91 ℃ |
| Hazen(Pt-Co): | ≤10 | Refactive Index: | 1.523 |
Description:
PTCS/PI is transparent liquid, can be used for phenyl intermediates and finished products production.
Production Application:
Raw materials for the synthesis of phenyl silicone oil and silicone resin, including molding,condensation type silicone, trapezoidal silicone, polysiloxane and other materials;
Preparation of organosilicone intermediates such as phenyloxilane;
For the preparation of halogen-freefalme retardant;
In the electronics industry as the active layer of organic thin film transistor substrate modification;
The terminal products are endowed with high and low temperature resistance, chemical stablility, radiation resistance, hihg regraction, hihg transmittance and other properties;
The terminal products are widely used in military, aerospace, unclear industry and other sophisticated fields.
Packaging information:
Steel drum (250kg/drum)
Customized package
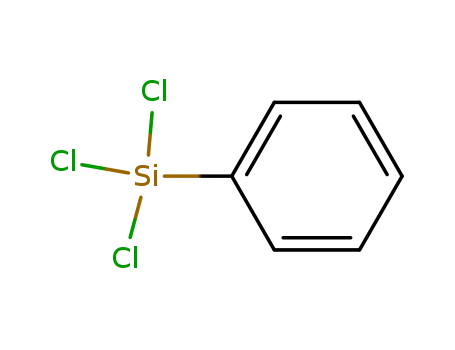
Manufacturers supply cost-effective and customizable Phenyltrichlorosilane(PTCS) 98-13-5
- Molecular Formula:C6H5Cl3Si
- Molecular Weight:211.55
- Appearance/Colour:Colorless transparent liquid
- Vapor Pressure:44.3Pa at 20℃
- Melting Point:-127 °C
- Refractive Index:n20/D 1.523(lit.)
- Boiling Point:201 °C at 760 mmHg
- Flash Point:87.5 °C
- PSA:0.00000
- Density:1.31 g/cm3
- LogP:2.54890
Phenyltrichlorosilane(Cas 98-13-5) Usage
|
Preparation |
The high-temperature condensation (HTC) technique proved to be very convenient and economical for the production of phenyltrichlorosilane and in recent years has become one of the main industrial techniques for its synthesis.The synthesis of phenyltrichlorosilane by HTC technique is conducted according to the reaction:HSiCI3 + C6H5CI →(HCI)→ C6H5SiCI3In this case, similarly to the vinyltrichlorosilane case, in the conditions of synthesis (600-640 °C) there is a secondary reaction of reduction, which forms silicon tetrachloride and benzene. However, if the process is conducted in the presence of an initiator (5% of diazomethane), it is possible to reduce the temperature down to 500-550 °C. Then the secondary reaction proceeds very slowly, which increases the yield of phenyltrichlorosilane by 1.5 times. To suppress the reduction process, one can also add benzene and silicon tetrachloride into the reactive mixture. It is also possible to boost the yield of phenyltrichlorosilane and increase the degree of the chlorobenzene transformation by using two subsequent reactors. |
|
Reactivity Profile |
Chlorosilanes, such as Phenyltrichlorosilane are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases. |
|
Health Hazard |
Highly toxic; may cause death or permanent injury after short inhalation exposure to small quantity. Chemical burns to all exposed membranes and tissues with severe tissue destruction. Inhalation -- lungs may fill up with fluid or throat may swell causing suffocation. Eyes -- damage to corneas may cause blindness. Delayed: after oral exposure stomach and intestines may perforate or be obstructed by scar tissue. |
|
Flammability and Explosibility |
Nonflammable |
|
Potential Exposure |
Phenyl trichlorosilane is used to make silicones for water repellants, insulating resins; heat resistant paints; and as a laboratory reagent |
|
Shipping |
UN1804 Phenyltrichlorosilane, Hazard class: 8; Labels: 8-Corrosive material. |
|
General Description |
A colorless liquid with a pungent odor. Flash point 91°F. Decomposed by moisture or water to hydrochloric acid with evolution of heat. Corrosive to metals and tissue. |
InChI:InChI=1/C6H5Si.3ClH/c7-6-4-2-1-3-5-6;;;/h1-5H;3*1H/q+3;;;/p-3
98-13-5 Relevant articles
Neutral-Eosin-Y-Photocatalyzed Silane Chlorination Using Dichloromethane
Fan, Xuanzi,Xiao, Pin,Jiao, Zeqing,Yang, Tingting,Dai, Xiaojuan,Xu, Wengang,Tan, Jin Da,Cui, Ganglong,Su, Hongmei,Fang, Weihai,Wu, Jie
supporting information, p. 12580 - 12584 (2019/08/16)
Chlorosilanes are versatile reagents in ...
Electrochemical properties of arylsilanes
Biedermann, Judith,Wilkening, H. Martin R.,Uhlig, Frank,Hanzu, Ilie
, p. 13 - 18 (2019/03/27)
In the past, the electrochemical propert...
Preparation method of phenyl chlorosilane
-
Paragraph 0053; 0054, (2019/07/04)
The invention discloses a preparation me...
A kind of preparation method of the midbody of entecavir, and intermediate
-
Paragraph 0386 - 0391; 0400; 0401, (2017/08/02)
The invention discloses Entecavir interm...
98-13-5 Process route
-
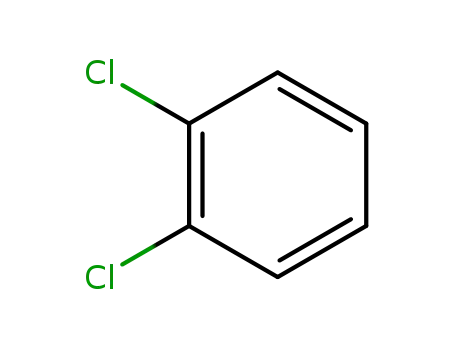
- 95-50-1
1,2-dichloro-benzene

-
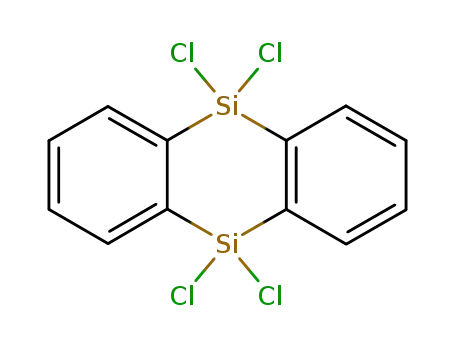
- 32962-41-7
9,9,10,10-Tetrachloro-9,10-dihydro-9,10-disilaanthracene

-
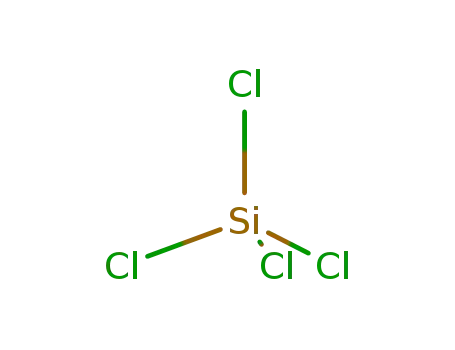
- 10026-04-7,53609-55-5
tetrachlorosilane

-
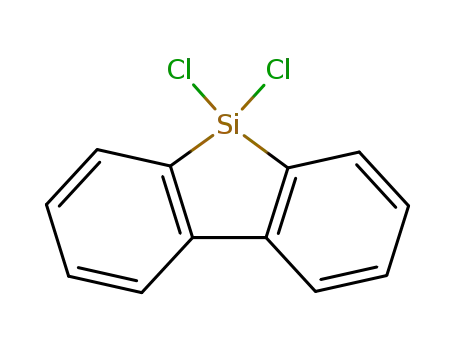
- 18030-58-5
9,9-dichloro-9-silafluorene

-
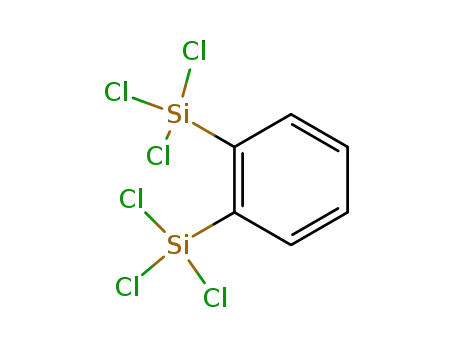
- 6838-77-3
o-bis(trichlorosilyl)benzene

-
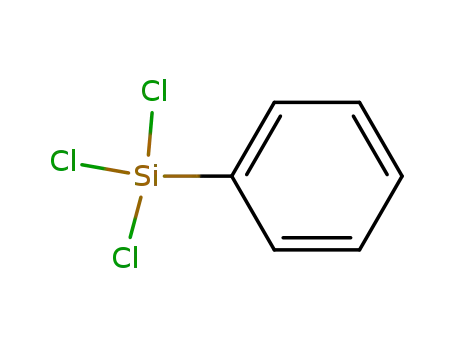
- 98-13-5
Phenyltrichlorosilane

-
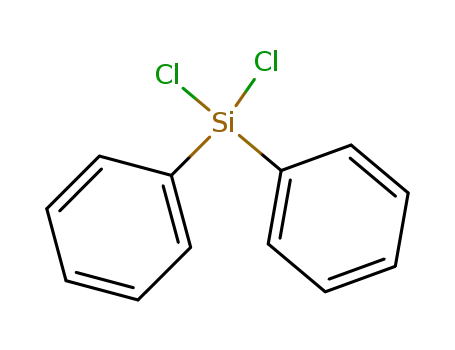
- 80-10-4
diphenylsilyl dichloride
| Conditions | Yield |
|---|---|
|
With copper; silicon; nickel(II) oxalate; cadmium(II) chloride; Product distribution; Heating; further catalysts;
|
47 % Chromat. 17 % Chromat. 8 % Chromat. 8 % Chromat. 1.2 % Chromat. 11 % Chromat. |
-
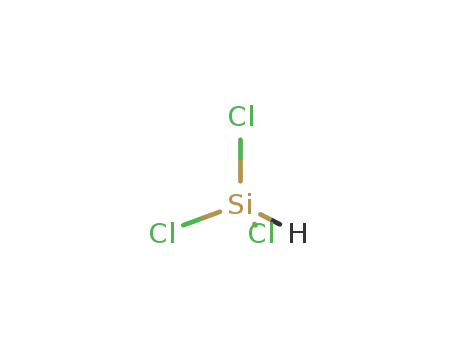
- 10025-78-2
trichlorosilane

-
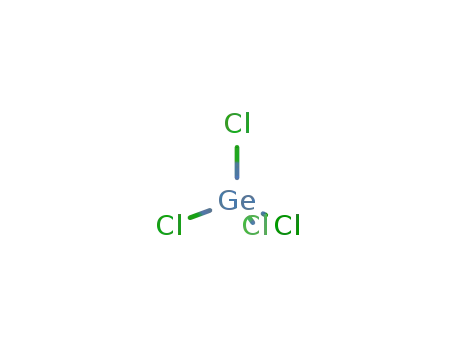
- 10038-98-9
germaniumtetrachloride

-
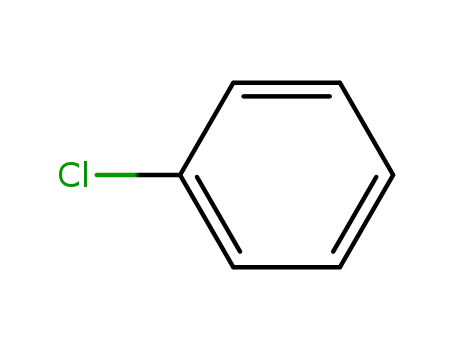
- 108-90-7
chlorobenzene

-

- 10026-04-7,53609-55-5
tetrachlorosilane

-
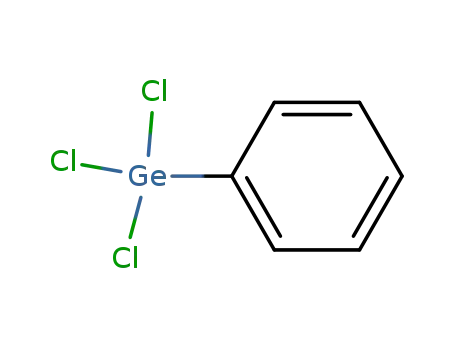
- 1074-29-9
trichlorophenylgermane

-

- 98-13-5
Phenyltrichlorosilane
| Conditions | Yield |
|---|---|
|
molar ratio of GeCl4, PhCl and silane 1:2:1, 580°, 30 sec (lower yields at 460 and 500°C and at different reactant ratio); condensation; gas. chromy.;
|
21% |
98-13-5 Upstream products
-
1623-99-0
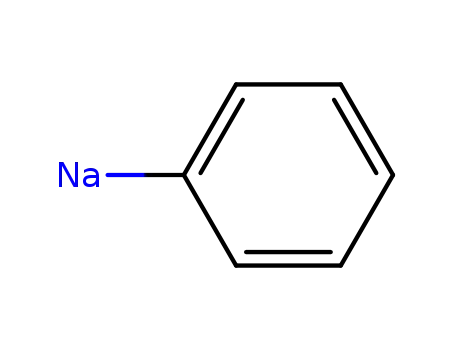
phenyl sodium
-
591-51-5
phenyllithium
-
108-90-7
chlorobenzene
-
587-85-9
diphenylmercury(II)
98-13-5 Downstream products
-
5700-37-8
phSi(Oet-2-Cl)3
-
17888-58-3
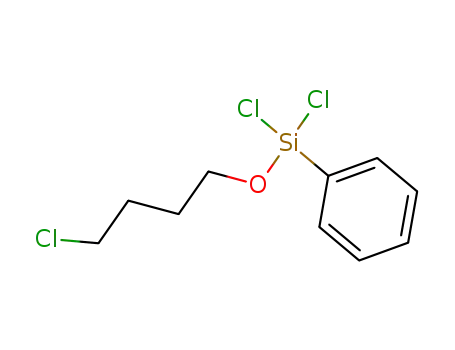
dichloro-(4-chloro-butoxy)-phenyl-silane
-
18557-51-2
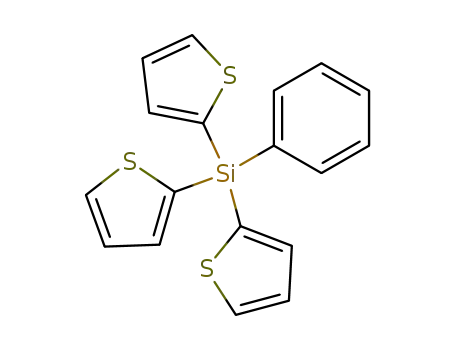
phenyl-tri-[2]thienyl-silane
-
18826-06-7
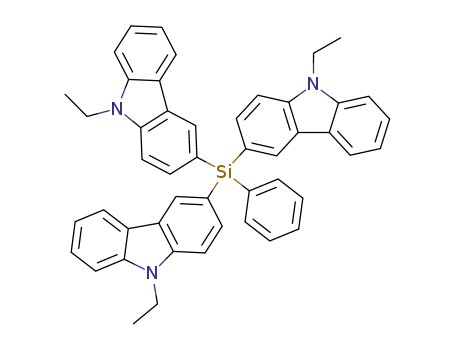
tris-(9-ethyl-carbazol-3-yl)-phenyl-silane
Relevant Products
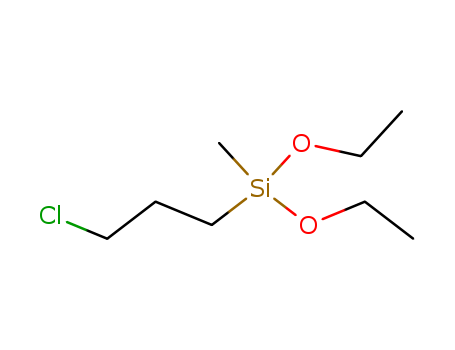
-
3-Chloropropylmethyldiethoxysilane
CAS:13501-76-3
-
PTFE Machined Components
CAS:9002-84-0
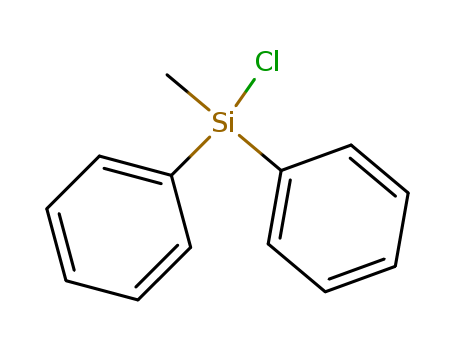
-
Chlorodiphenylmethylsilane(DPMCS)
CAS:144-79-6